 sales@zozen.com
sales@zozen.com
- WhatsApp: 0086-18861589035
-
 Contact
Us:
Contact
Us:
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water is more compatible with soap and extends the lifetime of plumbing. Water softening is usually achieved using lime softening or ion-exchange resins.
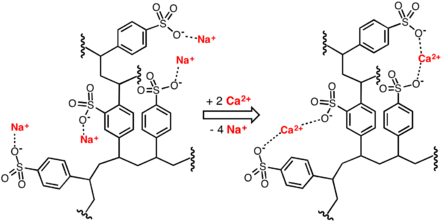
Idealized image of water softening process involving replacement of calcium ions in water with sodium ions donated by a cation-exchange resin.
Water softening methods
The most common means for removing water hardness rely on ion-exchange polymers or reverse osmosis. Other approaches include precipitation methods and sequestration by the addition of chelating agents.
- Ion-exchange resin devices
Conventional water-softening appliances intended for household use depend on an ion-exchange resin in which "hardness ions" - mainly Ca2+ and Mg2+ - are exchanged for sodium ions. Ion-exchange devices reduce the hardness by replacing magnesium and calcium (Mg2+ and Ca2+) with sodium or potassium ions (Na+ and K+).
- Types of ion-exchange materials
Ion exchange resins are organic polymers containing anionic functional groups to which the divalent cations (Ca++) bind more strongly than monovalent cations (Na+). Inorganic materials called zeolites also exhibit ion-exchange properties. These minerals are widely used in laundry detergents. Resins are also available to remove carbonate, bi-carbonate and sulphate ions which are absorbed and hydroxide ions released from the resin.

- Regeneration of ion-exchange resins
When all the available Na+ ions have been replaced with calcium or magnesium ions, the resin must be re-charged by eluting the Ca2+ and Mg2+ ions using a solution of sodium chloride or sodium hydroxide depending on the type of resin used.For anionic resins, regeneration typically uses a solution of sodium hydroxide (lye) or potassium hydroxide. The waste waters eluted from the ion-exchange column containing the unwanted calcium and magnesium salts are typically discharged to the sewage system.
Working Principle

Product View




I want to comment